Abstract
Background: CPX-351 (Vyxeos®) is a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a synergistic ratio. In a large randomized, open-label, multicenter, phase 3 study (NCT01696084) of CPX-351 versus conventional cytarabine/daunorubicin chemotherapy (7+3 regimen) in patients (pts) aged 60-75 years with newly diagnosed, high-risk/secondary AML, CPX-351 significantly improved overall survival (OS) and remission rates (Lancet JE, et al. J Clin Oncol. 2018). CPX-351 was approved by the US FDA in 2017 for the treatment of adults with newly diagnosed therapy-related AML or AML-MRC and is currently under review by the EMA. The WHO 2016 AML-MRC designation applies to AML pts who meet any of the following criteria: 1) a history of myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN), 2) an MDS-related cytogenetic abnormality, or 3) multilineage dysplasia in >50% of ≥2 cell lineages in the absence of NPM1 or biallelic CEBPA mutations. Pts with AML-MRC typically face a poor prognosis, with inferior outcomes in response to standard induction chemotherapy. A subgroup analysis of the phase 3 study was performed to specifically compare the efficacy and safety of CPX-351 versus 7+3 in pts with AML-MRC.
Methods: Pts enrolled in the phase 3 study who met the WHO 2008 AML-MRC classification were included in this subgroup analysis; however, pts with a history of MPN other than CMML or combined MDS/MPN and pts with only morphologic evidence of multilineage dysplasia were excluded from the phase 3 study. Pts were randomized 1:1 to receive up to 2 inductions with CPX-351 (100 units/m2 [cytarabine 100 mg/m2 + daunorubicin 44 mg/m2] on Days 1, 3, and 5 [2nd induction: Days 1 and 3]) or 7+3 (cytarabine 100 mg/m2/day continuously for 7 days [2nd induction: 5 days] + daunorubicin 60 mg/m2 on Days 1-3 [2nd induction: Days 1-2]). Pts achieving complete remission (CR) or CR with incomplete platelet or neutrophil recovery (CRi) could receive up to 2 consolidations with CPX-351 (65 units/m2 [cytarabine 65 mg/m2 + daunorubicin 29 mg/m2] on Days 1 and 3) or 5+2 (as in 2nd induction). Pts could receive hematopoietic cell transplantation (HCT) at the discretion of their treating physician.
Results: The study enrolled 309 pts, 246 of whom met the criteria for AML-MRC (CPX-351: n = 123; 7+3: n = 123). Of these 246 pts, 59.0% had antecedent MDS, 9.3% had antecedent CMML, and 31.7% had de novo AML with MDS karyotype. Baseline characteristics were similar between treatment arms (median age 68 years; 64.6% male; 11.0% with ECOG status of 2). A second induction cycle was received by 33.3% of pts in the CPX-351 arm and 37.8% of pts in the 7+3 arm.
Among pts with AML-MRC, CPX-351 was associated with a significant OS benefit versus 7+3 (median: 9.07 vs 5.95 months; HR = 0.70 [95% CI: 0.53-0.93]). CPX-351 was also associated with higher rates of CR+CRi (48.0% vs 32.5%; OR = 1.83 [95% CI: 1.09-3.09]), CR (37.4% vs 24.4%; OR = 1.80 [95% CI: 1.02-3.17]), and HCT (33.3% vs 24.4%; OR = 1.53 [95% CI: 0.86-2.74]). Among pts who received HCT, median OS was longer with CPX-351 versus 7+3 when calculated from the date of randomization (not reached vs 15.21 months; HR = 0.50 [95% CI: 0.26-0.99]) or date of HCT (not reached vs 10.68 months; HR = 0.48 [95% CI: 0.24-0.96]).
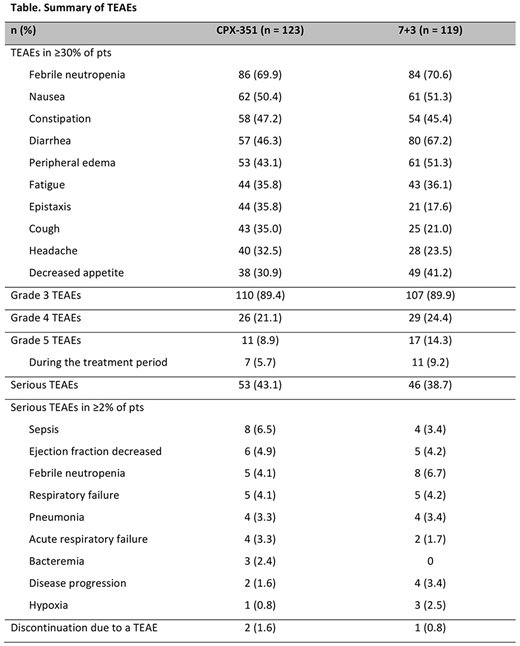
Early mortality rates in the CPX-351 and 7+3 arms, respectively, were 4.9% and 8.9% at Day 30 and 13.8% and 20.3% at Day 60. The treatment-emergent adverse event (TEAE) profile of CPX-351 in pts with AML-MRC was consistent with the overall study population and generally comparable between treatment arms (Table). Two pts treated with CPX-351 and 1 treated with 7+3 discontinued treatment due to a TEAE (cardiac failure [CPX-351], cardiomyopathy [CPX-351], and ejection fraction decreased [7+3]). Grade 5 TEAEs occurred in 8.9% and 14.3% of pts treated with CPX-351 and 7+3, respectively; those occurring in >1 pt in a treatment arm included sepsis (2.4% and 0.8%), disease progression (1.6% and 3.4%), multi-organ failure (0.8% and 1.7%), and respiratory failure (0.8% and 1.7%).
Conclusions: In this subgroup analysis, CPX-351 improved OS and remission rates compared with 7+3 in older adults with AML-MRC, while maintaining a similar safety profile. Importantly, CPX-351 is the first agent to be associated with prolonged OS compared with standard-of-care chemotherapy (7+3 regimen) in adults with newly diagnosed AML-MRC, which supported FDA approval in this population.
Ryan:AbbVie: Equity Ownership; University of Rochester: Patents & Royalties. Uy:Curis: Consultancy; GlycoMimetics: Consultancy. Cortes:Astellas Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Arog: Research Funding; Pfizer: Consultancy, Research Funding. Ritchie:Bristol-Myers Squibb: Research Funding; Incyte: Consultancy, Speakers Bureau; NS Pharma: Research Funding; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Astellas Pharma: Research Funding; Celgene: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau; Pfizer: Consultancy, Research Funding; ARIAD Pharmaceuticals: Speakers Bureau. Stuart:Sunesis Pharmaceuticals: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Agios: Research Funding; Astellas: Research Funding; Bayer AG: Research Funding; Celator/Jazz Pharmaceuticals: Research Funding; Incyte: Research Funding. Strickland:Sunesis Pharmaceuticals: Consultancy, Research Funding; Tolero Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Baxalta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Pharma: Consultancy. Bixby:GlycoMimetics: Research Funding. Kolitz:Magellan Health: Consultancy, Honoraria. Schiller:bluebird bio: Research Funding; Astellas Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding. Wieduwilt:Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Shire: Research Funding; Reata Pharmaceuticals: Equity Ownership; Leadiant: Research Funding; Amgen: Research Funding; Merck: Research Funding. Ryan:Jazz Pharmaceuticals: Employment, Other: Stock and stock options. Chiarella:Celator/Jazz Pharmaceuticals: Employment, Equity Ownership. Louie:Celator/Jazz Pharmaceuticals: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.